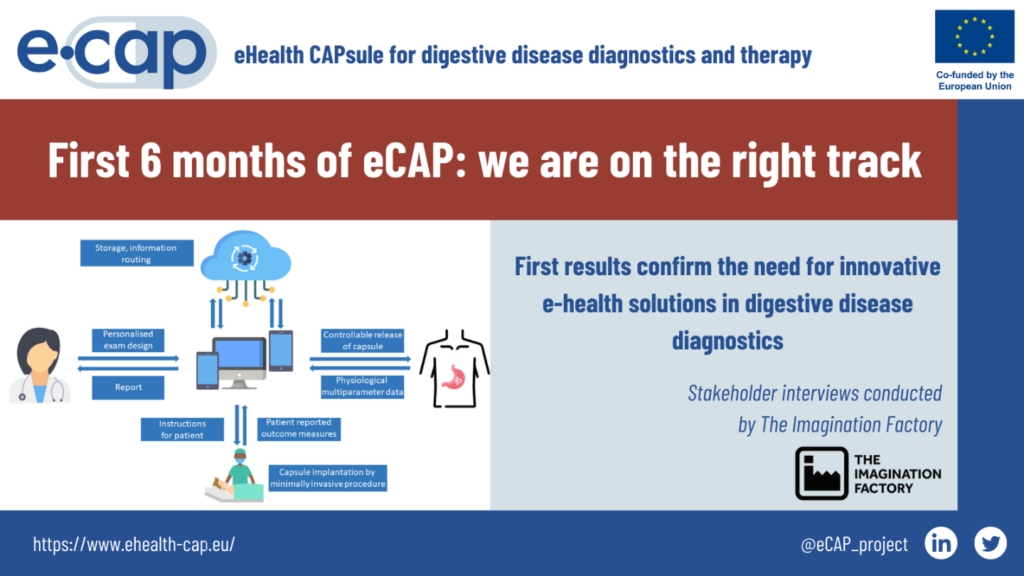
First six months of eCAP confirm the need for innovative e-health solutions in digestive disease diagnostics
November marks the end of the first six months of eCAP – a collaborative research and innovation project determined to tackle the worldwide gastroesophageal reflux (GERD) epidemic by developing a diagnostic smart capsule connected to an e-health platform. On the occasion of this milestone, the consortium partners met over a day-long teleconference to discuss project progress and plan next steps. ABIMI is supporting the dissemination and management activities within eCAP.
Over the course of the last half a year, the consortium has successfully completed the activities laying the groundwork for eCAP specifications and user design. Moreover, partners have undertaken the initial mapping of the regulatory landscape and established the methodology for the health technology assessment and cost-benefit analysis of eCAP. This scoping work is aimed at ensuring the project results in meaningful innovation catering to the needs of a growing patient population on the one hand, and health care systems stretched for resources on the other.
The stakeholder interviews conducted by Imagination Factory – work package leader for User Specifications – indicate eCAP consortium is on the right track. Both, patients and clinicians look forward to innovative technologies to increase the accuracy and ease of use of tests utilized for diagnosing gastroesophageal reflux. While patients would appreciate improved comfort, clinicians and nurses stress the need to cut the inefficiencies and costs related to the devices currently on the market. The stakeholder input gathered will feed into the mechanical design of the smart capsule, the work for which has been kicked off under the leadership of Tyndall National Institute.
At the M6 Progress Meeting, the consortium was also happy to welcome the contributions from its freshly formed External Advisory Board and Ethics Advisory Board. The Boards are composed of renowned experts from fields ranging from gastroenterology and medical devices to regulatory and ethics.
Read more about the meeting and project progress on eCAP website here.