ORGANTRANS: Consultation with EMA expert panel for regulatory approval
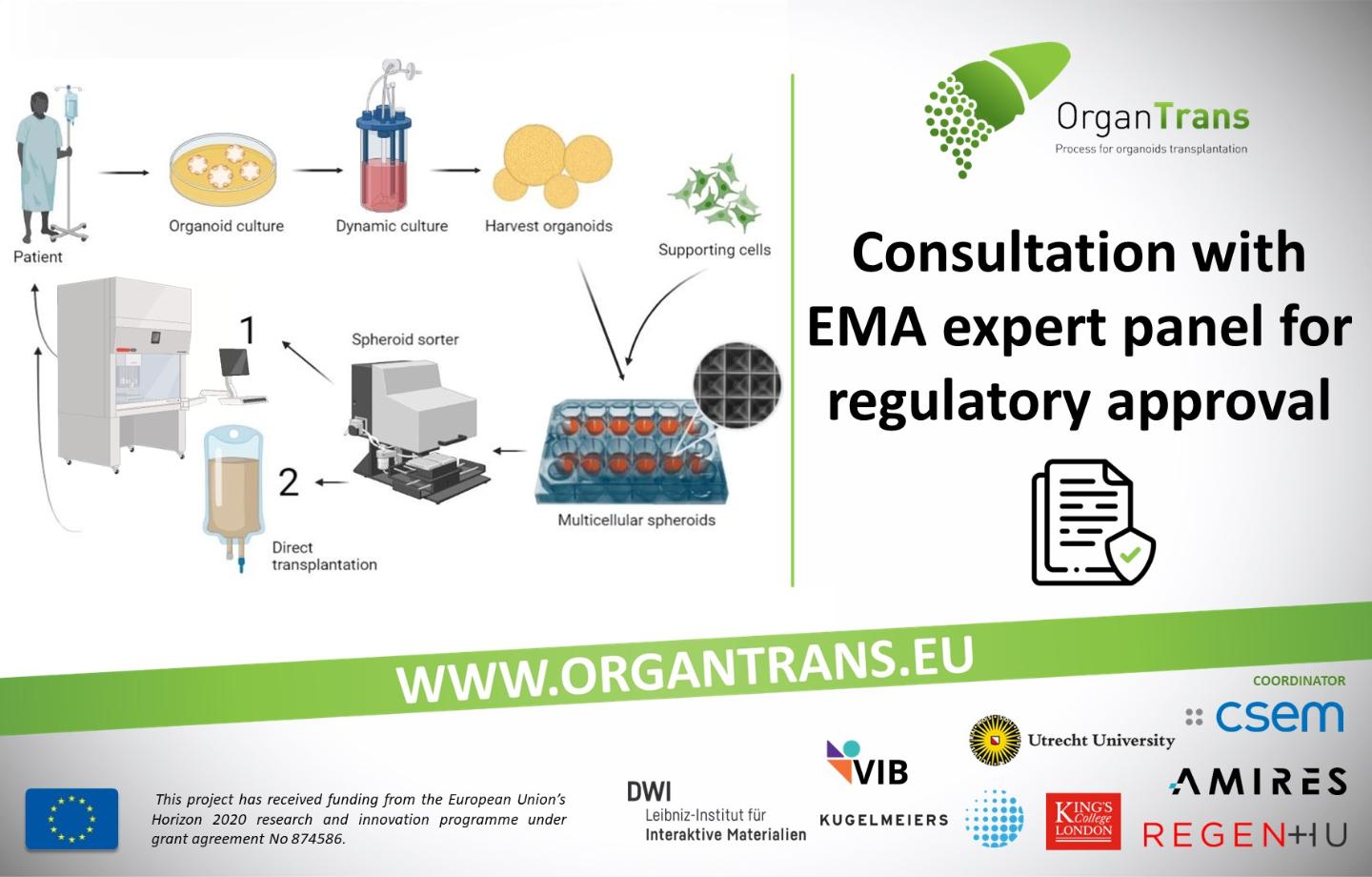
In translational science, where research is aimed at translating results in basic research into results that directly benefit humans, especially in a such complex project as the ORGANTRANS EU-funded project, it is inevitable to start communication with regulatory agencies as early as possible. Even at the project’s validation phase, ORGANTRANS representatives already contacted a multidisciplinary group called the Innovation Task Force of the European Medicines Agency (EMA) and met with more than 25 experts from all over Europe which included scientific, regulatory, and legal competencies. The fruitful discussion during this meeting will help in the further development of the ORGANTRANS product for the benefit of public health.
 |





